Medical Bioengineering SectionMedical Protein Engineering
Addives to supress irreversible thermal denaturation of disulfides containing protein
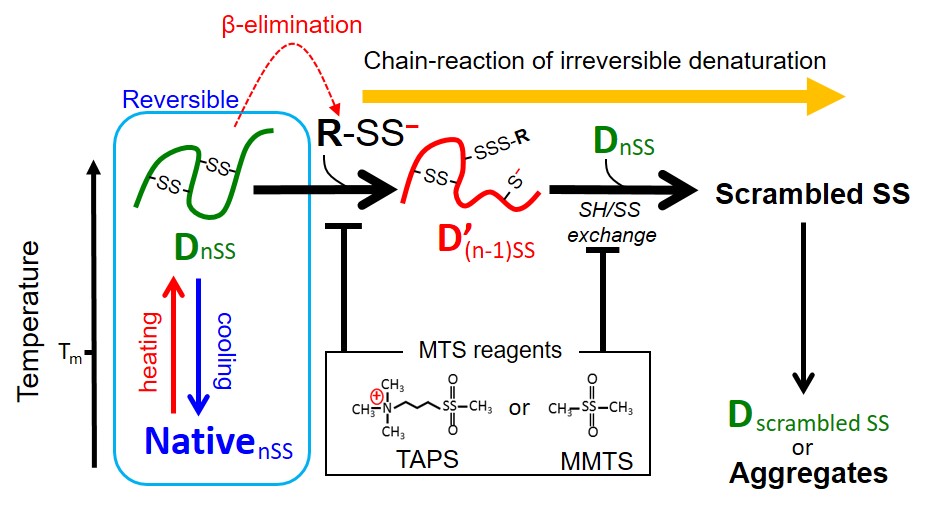
Proteins use disulfide (SS) bonds as “pillars” contributes higher conformational stability. Heat-induced SS bond breakages inside protein molecules generate highly reactive perthiole (R-SSH) that triggers SS-shuffling chain-reaction for irreversible denaturation.
We found this chain-reaction can be suppressed by adding methanethiosulfonate (MTS) type reagents that quickly eliminate perthiol groups, which are the starting point of the reaction. This methodology is now trying to medical applications.
Faculty
Medical Bioengineering Section Medical Protein Engineering
Assistant Professor MIYAMOTO Ai
